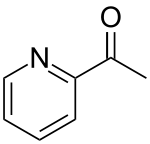
2-Acetylpyridine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Pyridin-2-yl)ethan-1-one | |
| Other names
1-(Pyridin-2-yl)ethanone 1-(2-Pyridinyl)ethanone Methyl 2-pyridyl ketone | |
| Identifiers | |
| 1122-62-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13648 |
| ECHA InfoCard | 100.013.051 |
| PubChem | 24901587 |
| |
| Properties | |
| C7H7NO | |
| Molar mass | 121.14 g·mol−1 |
| Density | 1.08 g/mL[1] |
| Melting point | 8 to 10 °C (46 to 50 °F; 281 to 283 K)[2] |
| Boiling point | 188 to 189 °C (370 to 372 °F; 461 to 462 K)[1] |
| Hazards | |
| Flash point | 73 °C (163 °F)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Acetylpyridine is an organic compound with the formula CH3COC5H4N. It is a viscous colorless liquid that is widely used as a flavoring substance. It is found in malt and produced by the Maillard reaction and by nixtamalization. It contributes to the flavor of corn tortillas, popcorn, and beer. [3]
The compound is prepared by acylation of 2-bromopyridine via the Grignard reagent.[4]
See also
References
- 1 2 3 Sigma Adrich
- ↑ ChemicalBook
- ↑ National Toxicology Program"Summary of Data for Chemical Selection"
- ↑ Trécourt, F.; Breton, G.; Bonnet, V.; Mongin, F.; Marsais, F.; Quéguiner, G., "New Syntheses of Substituted Pyridines via Bromine–Magnesium Exchange", Tetrahedron 2000, volume 56, pp. 1349-1360. doi:10.1016/S0040-4020(00)00027-2.
External links
This article is issued from Wikipedia - version of the 9/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.