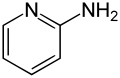
2-Aminopyridine
 | |
| Names | |
|---|---|
| IUPAC name
Pyridin-2-amine | |
| Other names
2-Pyridinamine; 2-Pyridylamine; α-Aminopyridine; α-Pyridylamine[1] | |
| Identifiers | |
| 504-29-0 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL21619 |
| ChemSpider | 10008 |
| ECHA InfoCard | 100.007.263 |
| PubChem | 10439 |
| |
| |
| Properties | |
| C5H6N2 | |
| Molar mass | 94.12 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 59 to 60 °C (138 to 140 °F; 332 to 333 K) |
| Boiling point | 210 °C (410 °F; 483 K) |
| >100%[1] | |
| Hazards | |
| Flash point | 68 °C; 154 °F; 341 K |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
200 mg/kg (rat, oral) 50 mg/kg (mouse, oral)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.5 ppm (2 mg/m3)[1] |
| REL (Recommended) |
TWA 0.5 ppm (2 mg/m3)[1] |
| IDLH (Immediate danger) |
5 ppm[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2-Aminopyridine is an organic compound with the formula H2NC5H4N. It is one of three isomeric aminopyridines. It is a colourless solid that is used in the production of the drugs piroxicam, sulfapyridine, tenoxicam, and tripelennamine. It is produced by the reaction of sodium amide with pyridine, the Chichibabin reaction.[3]
Structure
Although 2-hydroxypyridine exists in significant amounts as the pyridone tautomer, the related imine tautomer (HNC5H4NH) is less important for 2-aminopyridine.
References
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0026". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "2-Aminopyridine". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Shinkichi Shimizu, Nanao Watanabe, Toshiaki Kataoka, Takayuki Shoji, Nobuyuki Abe, Sinji Morishita, Hisao Ichimura "Pyridine and Pyridine Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_399
External links
This article is issued from Wikipedia - version of the 6/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.