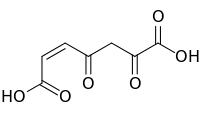
3-Maleylpyruvic acid
 | |
| Names | |
|---|---|
| IUPAC name
(Z)-4,6-Dioxohept-2-enedioic acid | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30859 |
| ChemSpider | 4444134 |
| PubChem | 5280494 6857405 (dianion) |
| |
| |
| Properties | |
| C7H6O6 | |
| Molar mass | 186.12 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Maleylpyruvic acid, or 3-maleylpyruvate, is a dicarboxylic acid formed by the oxidative ring opening of gentisic acid by gentisate 1,2-dioxygenase during the metabolism of tyrosine.[1] It is converted into 3-fumarylpyruvate by maleylpyruvate isomerase.[2]
References
This article is issued from Wikipedia - version of the 5/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.