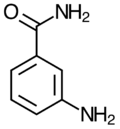
3-Aminobenzamide
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Aminobenzamide | |
Other names
| |
| Identifiers | |
| 3544-24-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:64042 |
| ChemSpider | 1583 |
| ECHA InfoCard | 100.020.534 |
| EC Number | 222-586-9 |
| PubChem | 1645 |
| |
| |
| Properties | |
| C7H8N2O | |
| Molar mass | 136.15 g·mol−1 |
| Appearance | Off-white powder |
| Density | 1.233g/cm3 |
| Melting point | 115 to 116 °C (239 to 241 °F; 388 to 389 K) |
| Boiling point | 329 °C (624 °F; 602 K) |
| log P | 0.33 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| GHS pictograms |  |
| GHS signal word | DANGER |
| H302, H315, H319, H335 | |
| P351, P338 | |
| EU classification (DSD) |
|
| R-phrases | R36/37/38 |
| S-phrases | S26-36 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1000mg/kg (oral, bird) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Aminobenzamide is a benzamide. It is an off-white powder and has the chemical formula C7H8N2O.
Preparation
3-Aminobenzamide can be prepared though the reduction of 3-nitrobenzamide by catalytic hydrogenation.[1]
Uses
3-Aminobenzamide is an inhibitor of poly ADP ribose polymerase (PARP), an enzyme responsible for DNA repair, transcription control, and programmed cell death.<ref="Purnell1980"/> When PARP is activated it rapidly uses up stores of nicotinamide adenine dinucleotide (NAD+) in the cell as it performs DNA repair. Low levels of NAD+ deplete the amount of ATP found in the cell which can lead to cell death.[2] The structure of 3-aminobenzamide is similar to that of NAD+ so it binds to PARP and prevents it from using up NAD+. PARP is often a target of cancer therapy and so 3-aminobenzamide could potentially be used as an anticancer drug.[3]
References
- ↑ Purnell, M. R; Whish, W. J. D. (1980). "Novel inhibitors of poly(ADP-ribose) synthetase". Biochem. J. 185 (3): 775–777. doi:10.1042/bj1850775. PMC 1161458
 . PMID 6248035.
. PMID 6248035. - ↑ 3-Aminobenzamide Product Information, Sigma-Aldrich, Accessed October 19, 2012
- ↑ Karlberg, T.; Hammarström, M.; Schütz, P.; Scensson, L.; Schüler, H. (2010). "Crystal Structure of the catalytic domain of human PARP2 in complex with PARP inhibitor ABT-888". Biochemistry. 49 (6): 1056–1058. doi:10.1021/bi902079y. PMID 20092359.
