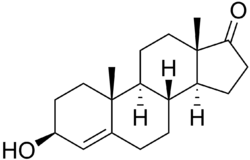
4-Dehydroepiandrosterone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
Androst-4-en-3β-ol-17-one | |
| Identifiers | |
| 571-44-8 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL1077603 |
| ChemSpider | 8123501 |
| PubChem | 9947889 |
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.43 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-Dehydroepiandrosterone (4-DHEA) is a steroid that is an isomer of 5-dehydroepiandrosterone.
4-DHEA has been prepared by laboratory synthesis.[1][2]
Synonyms
Synonyms for 4-dehydroepiandrosterone are:
3β-Hydroxy-4-androsten-17-one, 3β-hydroxyandrost-4-en-17-one, 3β-hydroxy-D4-androsten-17-one, 3β-hydroxyandrost-4-en-17-one, 3β-hydroxy-etioallocholan-4-en-17-one, and 4-androsten-3β-ol-17-one.
References
- ↑ Klimstra, Paul D.; Colton, F. B. Synthesis of 3β-hydroxyestr-4-en-17-one and 3β-hydroxyandrost-4-en-17-one. Steroids (1967), 10(4), 411-24.
- ↑ Ward, Margaret G.; Orr, James C.; Engel, Lewis L. A convenient synthesis of 3β-hydroxyandrost-4-en-17-one. Journal of Organic Chemistry (1965), 30(5), 1421-3.
This article is issued from Wikipedia - version of the 12/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.