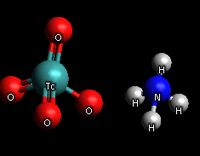
Ammonium pertechnetate
 | |
| Names | |
|---|---|
| IUPAC name
Ammonium pertechnetate | |
| Identifiers | |
| 34035-97-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7851725 |
| PubChem | 9577286 |
| |
| |
| Properties | |
| H4NO4Tc | |
| Molar mass | 180.04 g·mol−1 |
| Related compounds | |
| Other anions |
Ammonium nitrate |
| Other cations |
Sodium pertechnetate Potassium pertechnetate Calcium pertechnetate |
| Related compounds |
Perchloric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

Ammonium pertechnetate
Ammonium pertechnetate is a chemical compound with the formula NH4TcO4. It is the ammonium salt of pertechnetic acid. The most common form uses 99Tc.[1] The compound is readily soluble in aqueous solutions forming ammonium and pertechnetate ions.
It can be synthesized by the reaction of pertechnetic acid and ammonium nitrate:
- HTcO4 + NH4NO3 → NH4TcO4 + HNO3
It thermally decomposes under inert atmosphere at 700 °C to technetium dioxide:
NH4TcO4 → TcO2 + 2 H2O + 1/2 N2
References
This article is issued from Wikipedia - version of the 6/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.