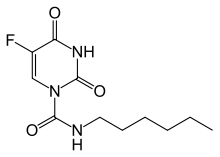
Carmofur
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | L01BC04 (WHO) |
| Identifiers | |
| |
| Synonyms | 1-hexylcarbamoyl-5-fluorouracil |
| CAS Number |
61422-45-5 |
| PubChem (CID) | 2577 |
| DrugBank |
DB09010 |
| ChemSpider |
2479 |
| UNII |
HA82M3RAB2 |
| ChEMBL |
CHEMBL460499 |
| ECHA InfoCard | 100.216.315 |
| Chemical and physical data | |
| Formula | C11H16FN3O3 |
| Molar mass | 257.261 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Carmofur (INN) or HCFU (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogue used as an antineoplastic agent. It is a derivative of fluorouracil.
Mechanism of action
The mechanism of action of carmofur is traditionally thought to be the generation of 5–FU.[1] However, carmofur is a highly potent acid ceramidase (AC) inhibitor.[1] Ceramide influences cancer cell survival, growth and death.[1] Inhibition of AC activity sensitizes tumor cells to the effects of antineoplastic agents and radiation.[1]
Uses
Carmofur, in its oral form, has also been used as adjuvant chemotherapy for curatively resected colorectal cancer patients. Trials and meta-analyses have confirmed that the drug is effective on patients with this cancer type, extending their survival.[2]
Adverse effects
As fluorouracil, carmofur has been known to induce leukoencephalopathy.[3][4][5]
References
- 1 2 3 4 Realini, Natalia; Solorzano, Carlos; Pagliuca, Chiara; Pizzirani, Daniela; Armirotti, Andrea; Luciani, Rosaria; Paola Costi, Maria; Bandiera, Tiziano; Piomelli, Daniele (8 January 2013). "Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity". Scientific Reports. 3 (1035). doi:10.1038/srep01035.
- ↑ Sakamoto, J; Hamada, C; Rahman, M; Kodaira, S; Ito, K; Nakazato, H; Ohashi, Y; Yasutomi, M (2005). "An Individual Patient Data Meta-analysis of Adjuvant Therapy with Carmofur in Patients with Curatively Resected Colon Cancer". Japanese Journal of Clinical Oncology. 35 (9): 536–544. doi:10.1093/jjco/hyi147. PMID 16155120.
- ↑ Yamada T, Okamura S, Okazaki T, et al. (June 1989). "Leukoencephalopathy following treatment with carmofur: a case report and review of the Japanese literature". Asia Oceania J Obstet Gynaecol. 15 (2): 161–8. PMID 2667512.
- ↑ Mizutani T (February 2008). "[Leukoencephalopathy caused by antineoplastic drugs]". Brain Nerve (in Japanese). 60 (2): 137–41. PMID 18306661.
- ↑ Baehring JM, Fulbright RK (May 2008). "Delayed leukoencephalopathy with stroke-like presentation in chemotherapy recipients". J Neurol Neurosurg Psychiatr. 79 (5): 535–9. doi:10.1136/jnnp.2007.123737. PMID 17682013.