Chromium acetate hydroxide
 | |
| Names | |
|---|---|
| IUPAC name
Chromium(III) acetate hydroxide | |
| Other names
Acetic acid of the chromium salt, Chromium(III)acetatehydroxide,Cr24%, Chromic acetate hydroxide, Chromium - acetic acid (3:7) dihydrate, Chromium Acetic Acid, Ccris 6345, | |
| Identifiers | |
| 39430-51-8 | |
| ChemSpider | 3383420 |
| ECHA InfoCard | 100.049.480 |
| EC Number | 254-447-3 |
| PubChem | 4172015 |
| Properties | |
| Cr3(OH)2(OOCCH3)7 | |
| Molar mass | 603.3 g/mol |
| Appearance | dark green solid |
| insoluble in acetone | |
| Hazards | |
| Main hazards | Xn, Harmful by inhalation, in contact with skin and if swallowed, Irritating to eyes, respiratory system and skin |
| Safety data sheet | MSDS |
| R-phrases | R20/21/22 R36/37/38 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
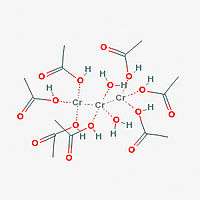
Chromium acetate hydroxide (Cr3(OH)2(OOCCH3)7) is a dark green powder. Chromium acetate hydroxide is non-ionic, is not soluble in acetone.[1]
Structure
Chromium acetate hydroxide contains three atoms of chromium, around which two hydroxide (OH) groups, and seven OOCCH3 monoanionic acetate ligands are arranged.
References
- ↑ The chemical book; chemicalbook.com, http://www.chemicalbook.com/ChemicalProductProperty_EN_CB9726110.htm, accessed February 2011
External links
| Wikimedia Commons has media related to Chromium(III) acetate hydroxide. |
- http://www.chemicalbook.com/ChemicalProductProperty_EN_CB9726110.htm
- http://www.chemicalbook.com/CAS%5Cmol%5C39430-51-8.mol
- https://web.archive.org/web/20120119193658/http://www.sigmaaldrich.com/catalog/ProductDetail.do?D7=0&N5=SEARCH_CONCAT_PNO|BRAND_KEY&N4=318108|ALDRICH&N25=0&QS=ON&F=SPEC
| Salts and the ester of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | ROAc | NH4OAc | AcOAc | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 | Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia - version of the 11/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.