Combretastatin A-4
 | |
| Names | |
|---|---|
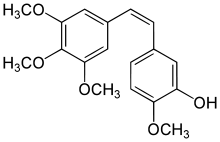
| IUPAC name
2-Methoxy-5-[(Z)-2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenol | |
| Other names
Combretastatin A4 CA-4 | |
| Identifiers | |
| 117048-59-6 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL67 |
| ChemSpider | 4508364 |
| ECHA InfoCard | 100.159.667 |
| PubChem | 5351344 |
| |
| |
| Properties | |
| C18H20O5 | |
| Molar mass | 316.34 g/mol |
| Melting point | 116 °C (241 °F; 389 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Combretastatin A-4 is a combretastatin and a stilbenoid. It can be isolated from Combretum caffrum, the Eastern Cape South African bushwillow tree or in Combretum leprosum, the mofumbo, a species found in Brazil.[2][3]
Function
Tubulin represents a potent target in cancer chemotherapy, given its role in cell division. Combretastatin is a naturally occurring well known tubulin polymerization inhibitor. Combretastatin A-4 comes in two isomers, only one of which inhibits polymerization.[3][4]
Derivatives
Combretastatin A-4 is the active component of combretastatin A-4 phosphate, a prodrug designed to damage the vasculature (blood vessels) of cancer tumors causing central necrosis. A large number of synthetic derivatives have been reported, including beta-lactam based compounds.[5]
See also
- Ombrabulin, a combretastatin A-4 derivative in clinical trials for treatment of cancer
References
- ↑ Pettit, G. R.; Sheo Bux Singh Boyd; M. R. Hamel, E. (1995), "Antineoplastic Agents. 291. Isolation and Synthesis of Combretastatins A-4, A-5, and A-6", Journal of Medicinal Chemistry, 38: 1666–1672, doi:10.1021/jm00010a011
- ↑ Determination of Combretastatin A-4 in Combretum leprosum. SCN Queiroz, MR Assalin, S Nobre, IS Melo, RM Moraes, VL Ferracini and AL Cerdeira, Planta Med, 2010, volume 76, pages 53, doi:10.1055/s-0030-1251815
- 1 2 Gill, Rupinder; Kaur, Ramandeep; Kaur, Gurneet; Rawal, Ravindra; Shah, Anamik; Bariwal, Jitender. "A Comprehensive Review on Combretastatin Analogues as Tubulin Binding Agents". Current Organic Chemistry. 18 (19): 2462–2512. doi:10.2174/138527281819141028114428.
- ↑ "Colourful chemotherapy". The Economist. July 11, 2015. ISSN 0013-0613. Retrieved 2016-06-19.
- ↑ O'Boyle, N; Miriam Carr; Lisa M. Greene; Orla Bergin; Seema M. Nathwani; Thomas McCabe; David G. Lloyd; Daniela M Zisterer; Mary J. Meegan (2010). "Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents.". Journal of Medicinal Chemistry. 53 (24): 8569–8584. doi:10.1021/jm101115u. PMID 21080725.