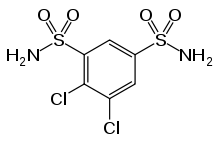
Diclofenamide
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601233 |
| ATC code | S01EC02 (WHO) |
| Pharmacokinetic data | |
| Protein binding | 55% |
| Identifiers | |
| |
| CAS Number |
120-97-8 |
| PubChem (CID) | 3038 |
| IUPHAR/BPS | 6807 |
| DrugBank |
DB01144 |
| ChemSpider |
2930 |
| UNII |
VVJ6673MHY |
| KEGG |
D00518 |
| ChEBI |
CHEBI:101085 |
| ChEMBL |
CHEMBL17 |
| ECHA InfoCard | 100.004.037 |
| Chemical and physical data | |
| Formula | C6H6Cl2N2O4S2 |
| Molar mass | 305.16 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 228.5 °C (443.3 °F) |
| |
| |
| | |
Diclofenamide (or dichlorphenamide) is a sulfonamide and a carbonic anhydrase inhibitor of the meta-disulfamoylbenzene class.
Uses
Diclofenamide is used to treat glaucoma[1][2] and therapy-resistant epilepsy.[3]
References
- ↑ International Drug Names: Diclofenamide
- ↑ Kanski, J. J. (1968). "Carbonic anhydrase inhibitors and osmotic agents in glaucoma. Carbonic anhydrase inhibitors". The British journal of ophthalmology. 52 (8): 642–643. doi:10.1136/bjo.52.8.642. PMC 506660
 . PMID 5724852.
. PMID 5724852. - ↑ Rucquoy, M.; Sorel, L. (1978). "Diclofenamide in the treatment of therapy-resistant epilepsy". Acta Neurologica Belgica. 78 (3): 174–182. PMID 352085.
This article is issued from Wikipedia - version of the 11/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.