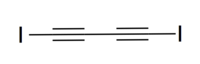
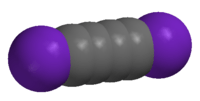
Diiodobutadiyne
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,4-Diiodobuta-1,3-diyne | |
Other names
| |
| Identifiers | |
| 53214-97-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 126166 |
| PubChem | 143018 |
| |
| |
| Properties | |
| C4I2 | |
| Molar mass | 301.85 g·mol−1 |
| Appearance | White solid |
| Solubility in Hexanes | soluble |
| Structure | |
| Linear | |
| Explosive data | |
| Shock sensitivity | Sensitive - may explode if struck |
| Related compounds | |
| Related compounds |
Diacetylene, PIDA |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,4-Diiodobuta-1,3-diyne is a small molecule related to diacetylene. It is used in the creation of the polymer poly(diiododiacetylene) (PIDA) by undergoing 1,4 polymerization.[1] It is light sensitive and explosive if stored out of solution as a dry solid. It will undergo random 1,2 and 1,4 polymerization, as well as decomposition in solution if kept over an extended period of time, having a half life of just about two weeks.[2]
References
- ↑ Sun, A.; Lauher, J.W.; Goroff, N.S. (2006), "Preparation of Poly(Diiododiacetylene), an Ordered Conjugated Polymer of Carbon and Iodine", Science, 312 (5776): 1030–1034, Bibcode:2006Sci...312.1030S, doi:10.1126/science.1124621, PMID 16709780
- ↑ Luo, Liang; Wilhelm, Christopher; Sun, Aiwu; Grey, Clare P.; Lauher, Joseph W.; Goroff, Nancy S. (2008), "Poly(Diiododiacetylene): Preparation, Isolation, and Full Characterization of a Very Simple Poly(diacetylene)", Journal of the American Chemical Society, 103: 7702–7709, doi:10.1021/ja8011403
This article is issued from Wikipedia - version of the 2/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.