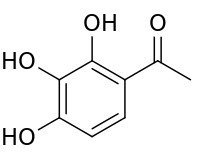
Gallacetophenone
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(2,3,4-Trihydroxyphenyl)ethan-1-one | |
| Other names
1-(2,3,4-Trihydroxyphenyl)ethanone Alizarin Yellow C Galloacetophenone 2',3',4'-Trihydroxyacetophenone | |
| Identifiers | |
| 528-21-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10256 |
| ECHA InfoCard | 100.007.665 |
| PubChem | 10706 |
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.15 g·mol−1 |
| Melting point | 171 to 172 °C (340 to 342 °F; 444 to 445 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Gallacetophenone is the acetyl derivative of pyrogallol. It can be synthesized from pyrogallol using zinc chloride and acetic anhydride.[1]
References
This article is issued from Wikipedia - version of the 11/13/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.