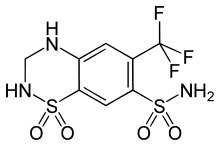
Hydroflumethiazide
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | C03AA02 (WHO) |
| Identifiers | |
| |
| CAS Number |
135-09-1 |
| PubChem (CID) | 3647 |
| IUPHAR/BPS | 7197 |
| DrugBank |
DB00774 |
| ChemSpider |
3521 |
| UNII |
501CFL162R |
| KEGG |
D00654 |
| ChEMBL |
CHEMBL1763 |
| ECHA InfoCard | 100.004.704 |
| Chemical and physical data | |
| Formula | C8H8F3N3O4S2 |
| Molar mass | 331.29 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Hydroflumethiazide (or Saluron) is a diuretic.
Synthesis
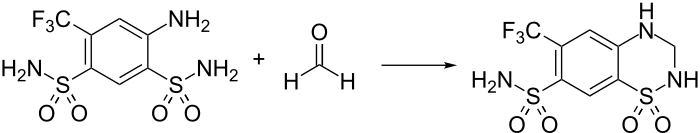
Hydroflumethiazide synthesis:[1][2][3] Numerous patents, e.g., Lund et al., U.S. Patent 3,254,076 (1966 to Lövens Kemiske Fabrik).
See also
References
- ↑ Holdrege, C. T.; Babel, R. B.; Cheney, L. C. (1959). "Synthesis of Trifluoromethylated Compounds Possessing Diuretic Activity1". Journal of the American Chemical Society. 81 (18): 4807. doi:10.1021/ja01527a015.
- ↑ Close, W. J.; Swett, L. R.; Brady, L. E.; Short, J. H.; Vernsten, M. (1960). "Synthesis of Potential Diuretic Agents. I. Derivatives of 7-Sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-Dioxide". Journal of the American Chemical Society. 82 (5): 1132. doi:10.1021/ja01490a028.
- ↑ Novello, F. C.; Bell, S. C.; Abrams, E. L. A.; Ziegler, C.; Sprague, J. M. (1960). "Diuretics: 1,2,4-Benzothiadiazine-1,1-dioxides". The Journal of Organic Chemistry. 25 (6): 970. doi:10.1021/jo01076a028.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.