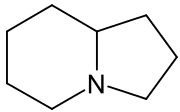
Indolizidine
 | |
| Names | |
|---|---|
| IUPAC name
Octahydroindolizine | |
| Other names
δ-Coniceine; 1-Azabicyclo[4.3.0]nonane | |
| Identifiers | |
| 13618-93-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 24347 |
| ECHA InfoCard | 100.033.716 |
| PubChem | 26136 |
| |
| |
| Properties | |
| C8H15N | |
| Molar mass | 125.22 g·mol−1 |
| Density | 0.8956 g/cm3 (20 °C)[1] |
| Boiling point | 159 to 160 °C (318 to 320 °F; 432 to 433 K)[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indolizidine is a heterocyclic chemical compound that forms the central core of the indolizidine alkaloids such as swainsonine and castanospermine.
See also
References
- ↑ Skvortsov, I. M.; Zadumina, E. A.; Ponomarev, A. A. (1965). "1-Azabicycles. IV. Catalytic synthesis of 1-azabicyclo[4.3.0]nonanes and 1-azabicyclo[5.3.0]decanes". Khimiya Geterotsiklicheskikh Soedinenii. 6: 864–868.
- ↑ Lavagnino, Edward R.; Chauvette, Robert R.; Cannon, William N.; Kornfeld, Edmund C. (1960). "Conidine—Synthesis, Polymerization and Derivatives". Journal of the American Chemical Society. 82 (10): 2609–2613. doi:10.1021/ja01495a054.
This article is issued from Wikipedia - version of the 9/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.