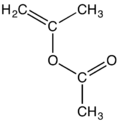
Isopropenyl acetate
 | |
| Names | |
|---|---|
| Other names
1-Methylvinyl acetate | |
| Identifiers | |
| 108-22-5 | |
| Properties | |
| C5H7O2 | |
| Molar mass | 100.12 |
| Appearance | colorless liquid |
| Density | 0.9090 g/cm3 (20 ºC) |
| Melting point | -92.9 °C |
| Boiling point | 97 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isopropenyl acetate is an organic compound, which is the acetate ester of the enol tautomer of acetone. This a colorless liquid is significant as the principal precursor to acetylacetone.
Preparation and reactions
Isopropenyl acetate is prepared by treating acetone with ketene.[1] Upon heating over a metal surface, isopropenyl acetate rearranges to acetylacetone.
References
- ↑ Raimund Miller, Claudio Abaecherli, Adel Said, Barry Jackson "Ketenes" in Ullmann's Encyclopedia of Industrial Chemistry, 2001, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a15_063
This article is issued from Wikipedia - version of the 10/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.