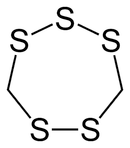
Lenthionine
Not to be confused with Lanthionine or Lanthanide.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2,3,5,6-Pentathiepane | |||
| Other names
1,2,3,5,6-Pentathiacycloheptane | |||
| Identifiers | |||
| 292-46-6 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:6408 | ||
| ChemSpider | 60844 | ||
| KEGG | C08382 | ||
| PubChem | 67521 | ||
| |||
| |||
| Properties | |||
| C2H4S5 | |||
| Molar mass | 188.38 g/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Lenthionine is a cyclic organosulfur compound found in shiitake mushrooms and partly responsible for their flavor.[1] The mechanism of its formation is unclear, but it probably involves the enzyme C–S lyase.
Besides giving flavor to shiitake mushrooms, lenthionine inhibits platelet aggregation, so it is a promising treatment for thrombosis.[2] Other organosulfur compounds found in garlic have a similar effect.
References
- ↑ Block, Eric; Deorazio, Russell (1994). "Chemistry in a salad bowl: Comparative organosulfur chemistry of garlic, onion and shiitake mushrooms" (PDF). Pure & Appl. Chem. 66 (10–11): 2205–2206. doi:10.1351/pac199466102205.
- ↑ Shibuya, T.; Shimada, S.; Sakurai, H.; Kumagai, H. (2005). "Mechanism of inhibition of platelet aggregation by lenthionine, a flavor component from shiitake mushroom". IFT Annual Meeting: Presentation 54G–9.
This article is issued from Wikipedia - version of the 6/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.