Medrysone
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a606003 |
| Pregnancy category |
|
| Routes of administration | Eye drops |
| ATC code | S01BA08 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
2668-66-8 |
| PubChem (CID) | 247839 |
| IUPHAR/BPS | 7086 |
| DrugBank |
DB00253 |
| ChemSpider |
216968 |
| UNII |
D2UFC189XF |
| KEGG |
D02289 |
| ChEMBL |
CHEMBL1201173 |
| Chemical and physical data | |
| Formula | C22H32O3 |
| Molar mass | 344.488 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
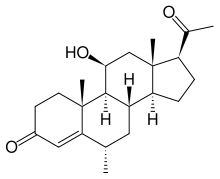
Medrysone (INN, USAN), also known as 6α-Methyl-11β-hydroxyprogesterone or 11β-hydroxy-6α-methylpregn-4-ene-3,20-dione, is a corticosteroid that has been used in optometry, and in ophthalmology for the treatment of eye inflammations.[1][2] It has been discontinued in the US.[2]
References
This article is issued from Wikipedia - version of the 10/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.