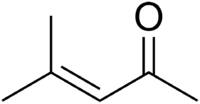
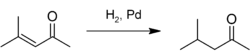Mesityl oxide
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-methylpent-3-en-2-one | |
| Other names
Mesityl oxide Isobutenyl methyl ketone Methyl isobutenyl ketone Isopropylidene acetone | |
| Identifiers | |
| 141-79-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 8526 |
| ECHA InfoCard | 100.005.002 |
| RTECS number | SB4200000 |
| |
| |
| Properties | |
| C6H10O | |
| Molar mass | 98.15 g·mol−1 |
| Appearance | Oily, colorless to light-yellow liquid[1] |
| Odor | peppermint- or honey-like[1] |
| Density | 0.858 g/cm3 |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 129.5 °C (265.1 °F; 402.6 K) |
| 3% (20°C)[1] | |
| Solubility in other solvents | Soluble in most organic solvents |
| Vapor pressure | 9 mmHg (20°C)[1] |
| Refractive index (nD) |
1.442 |
| Hazards | |
| Main hazards | flammable |
| R-phrases | R10 R20/21/22 |
| S-phrases | S25 |
| Flash point | 31 °C; 87 °F; 304 K [1] |
| Explosive limits | 1.4%-7.2%[1] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1120 mg/kg (rat, oral) 1000 mg/kg (rabbit, oral) 710 mg/kg (mouse, oral)[2] |
| LC50 (median concentration) |
1000 mg/m3 (rat, 4 hr) 9000 mg/m3 (rat, 4 hr) 10,000 mg/m3 (mouse, 2 hr) 2000 mg/m3 (guinea pig, 7 hr)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 25 ppm (100 mg/m3)[1] |
| REL (Recommended) |
TWA 10 ppm (40 mg/m3)[1] |
| IDLH (Immediate danger) |
1400 ppm[1] |
| Related compounds | |
| Related compounds |
diacetone alcohol acetone, benzylideneacetone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Mesityl oxide is a α,β-Unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a strong cat urine odor.[3]
Synthesis
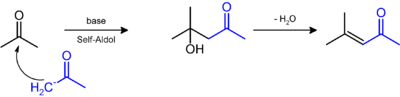
It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.[4]
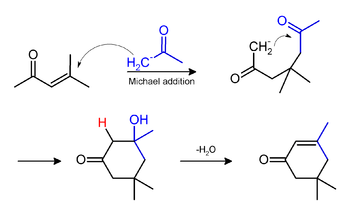
Isophorone may be formed under the same conditions of mesityl oxide production, by a Michael addition. The yields of mesityl oxide and isophorone may vary according to reaction conditions during synthesis:
Uses
Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:
References
- 1 2 3 4 5 6 7 8 9 "NIOSH Pocket Guide to Chemical Hazards #0385". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "Mesityl oxide". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Merck Index, 11th Edition, 5811
- ↑ J. B. Conant and Neal Tuttle (1941). "Mesityl oxide". Org. Synth.; Coll. Vol., 1, p. 345
External links
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.