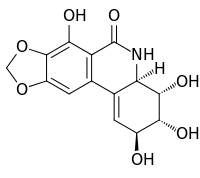
Narciclasine
 | |
| Names | |
|---|---|
| IUPAC name
(2S-(2α,3β,4β,4aβ))-3,4,4a,5-Tetrahydro-2,3,4,7-tetrahydroxy-(1,3)dioxolo(4,5-j)phenanthridin-6(2H)-one | |
| Other names
BRN 1087400, Lycoricidin-A, Lycoricidinol, NSC 266535 | |
| Identifiers | |
| 29477-83-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 65310 |
| ECHA InfoCard | 100.214.093 |
| KEGG | C08533 |
| PubChem | 72376 |
| |
| |
| Properties | |
| C14H13NO7 | |
| Molar mass | 307.26 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Narciclasine is a toxic alkaloid found in various Amaryllidaceae species.[1]
References
- ↑ Kornienko A, Evidente A (2008). "Chemistry, biology, and medicinal potential of narciclasine and its congeners". Chem Rev. 108 (6): 1982–2014. doi:10.1021/cr078198u. PMC 2856661
 . PMID 18489166.
. PMID 18489166.
Bibliography
- Gwendoline Van Goietsenoven; Véronique Mathieu; Florence Lefranc; Alexander Kornienko; Antonio Evidente; Robert Kiss (March 2013). "Narciclasine as well as other Amaryllidaceae Isocarbostyrils are Promising GTP-ase Targeting Agents against Brain Cancers". Medicinal Research Reviews. 33 (2): 439–455. doi:10.1002/med.21253.
This article is issued from Wikipedia - version of the 6/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.