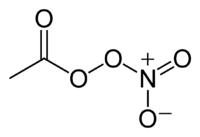
Peroxyacetyl nitrate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
nitroethaneperoxoate | |
| Systematic IUPAC name
ethanoic nitric peroxyanhydride | |
| Other names
PAN peroxyacetyl nitrate α-oxoethylperoxylnitrate | |
| Identifiers | |
| 2278-22-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 15907 |
| ECHA InfoCard | 100.017.187 |
| EC Number | 218-905-6 |
| PubChem | 16782 |
| |
| |
| Properties | |
| C2H3NO5 | |
| Molar mass | 121.05 g mol−1 |
| 1.46 × 10 5 mg l−1 at 298 K | |
| log P | −0.19 |
| Vapor pressure | 29.2 mmHg at 298 K |
| Henry's law constant (kH) |
0.000278 m3 atm mol−1 at 298 K |
| Atmospheric OH rate constant |
10−13 cm3 molecule−1 s−1 at 298 K |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Peroxyacetyl nitrate is a peroxyacyl nitrate. It is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance.
Peroxyacetyl nitrate, or PAN, is an oxidant more stable than ozone. Hence, it is better capable of long-range transport than ozone. It serves as a carrier for oxides of nitrogen (NOx) into rural regions and causes ozone formation in the global troposphere.
External links
This article is issued from Wikipedia - version of the 9/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.