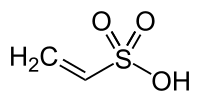
Vinylsulfonic acid
 | |
| Names | |
|---|---|
| IUPAC name
ethenesulfonic acid | |
| Identifiers | |
| 1184-84-5 3039-83-6 (sodium salt) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56254 |
| ECHA InfoCard | 100.013.342 |
| PubChem | 62474 |
| |
| |
| Properties | |
| C2H4O3S | |
| Molar mass | 108.11 g·mol−1 |
| Appearance | colouless liquid |
| Boiling point | 95 °C (203 °F; 368 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vinylsulfonic acid is an organosulfur compound. It is a colorless liquid that tends to polymerize to polyvinylsulfonic acid. It is prepared by dehydration of isethionic acid:[1]
- HOC2H4SO3H → CH2CHSO3H + H2O
More easily handled than the acid is the salt, sodium vinylsulfonate, which is commercially supplied as an aqueous solution.
References
- ↑ Kosswig, Kurt (2000). "Sulfonic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a25_503. ISBN 3-527-30673-0.
This article is issued from Wikipedia - version of the 12/17/2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.