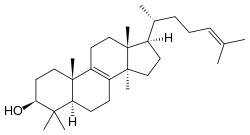
Lanosterol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
lanosta-8,24-dien-3-ol | |
| Identifiers | |
| 79-63-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16521 |
| ChEMBL | ChEMBL225111 |
| ChemSpider | 216175 |
| ECHA InfoCard | 100.001.105 |
| 2746 | |
| MeSH | Lanosterol |
| PubChem | 246983 |
| UNII | 1J05Z83K3M |
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.71 g/mol |
| Melting point | 138 to 140 °C (280 to 284 °F; 411 to 413 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungi steroids are derived. By contrast plant steroids are produced via cycloartenol.[1]
Role in creation of steroids
Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol.
Recent research suggests that lanosterol might be instrumental in prevention of formation of cataracts in mammals.[2]
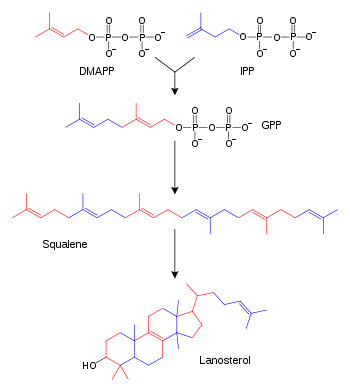
Biosynthesis
| Description | Illustration | Enzyme |
| Two molecules of farnesyl pyrophosphate condense with reduction by NADPH to form squalene | | squalene synthase |
| Squalene is oxidized to 2,3-oxidosqualene (squalene epoxide) | 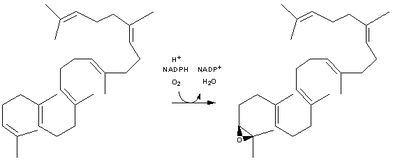 | squalene monooxygenase |
| 2,3-Oxidosqualene is converted to a protosterol cation and finally to lanosterol | 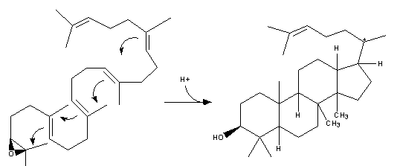 | lanosterol synthase |
| (step 2) | 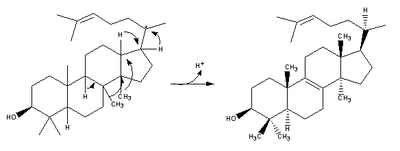 | (step 2) |
Clinical significance
Preliminary studies in dogs and rabbits have shown that lanosterol can prevent and even reverse cataract formation.[2][3] However, an attempt to replicate these results in age-related cataractous human lens nuclei removed during manual small incision cataract surgery by immersing them in lanosterol solution and incubating them for 6 days according to the method of Zhao et al.,[2] failed to reverse nuclear opacity.[4]
See also
References
- ↑ Schaller, Hubert (May 2003). "The role of sterols in plant growth and development". Progress in Lipid Research. 42 (3): 163–175. doi:10.1016/S0163-7827(02)00047-4.
- 1 2 3 Ling Zhao; Xiang-Jun Chen; Jie Zhu; Yi-Bo Xi; Xu Yang; Li-Dan Hu; Hong Ouyang; Sherrina H. Patel; Xin Jin; Danni Lin; Frances Wu; Ken Flagg; Huimin Cai; Gen Li; Guiqun Cao; Ying Lin; Daniel Chen; Cindy Wen; Christopher Chung; Yandong Wang; Austin Qiu; Emily Yeh; Wenqiu Wang; Xun Hu; Seanna Grob; et al. (July 2015). "Lanosterol reverses protein aggregation in cataracts". Nature. doi:10.1038/nature14650.
- ↑ Groß, M. (2015), Aggregate aufgelöst. Chemie in unserer Zeit. doi:10.1002/ciuz.201580036
- ↑ Shanmugam, P. M., Barigali, A., Kadaskar, J., Borgohain, S., Mishra, D. K. C., Ramanjulu, R., & Minija, C. K. (2015). Effect of lanosterol on human cataract nucleus. Indian journal of ophthalmology, 63(12), 888-890 doi:10.4103/0301-4738.176040
- E. J. Corey, W. E. Russey, P. R. Ortiz de Montellano (1966). "2,3-Oxidosqualene, an Intermediate in the Biological Synthesis of Sterols from Squalene". Journal of the American Chemical Society. 88 (20): 4750–4751. doi:10.1021/ja00972a056. PMID 5918046.
- I. Abe; M. Rohmer; G. D. Prestwich (1993). "Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes". Chemical Reviews. 93 (6): 2189–2206. doi:10.1021/cr00022a009.
- A. Eschenmoser, L. Ruzicka, O. Jeger, D. Arigoni (1955). "Zur Kenntnis der Triterpene. 190. Mitteilung. Eine stereochemische Interpretation der biogenetischen Isoprenregel bei den Triterpenen". Helvetica Chimica Acta. 38 (7): 1890–1904. doi:10.1002/hlca.19550380728.