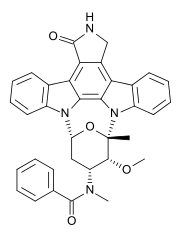
Midostaurin
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 4'-N-benzoylstaurosporine |
| CAS Number | 120685-11-2 |
| PubChem (CID) | 9829523 |
| IUPHAR/BPS | 5702 |
| ChemSpider | 8005258 |
| UNII |
ID912S5VON |
| ChEMBL | CHEMBL608533 |
| Chemical and physical data | |
| Formula | C35H30N4O4 |
| Molar mass | 570.637 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Midostaurin (PKC412) is a multi-target protein kinase inhibitor being investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocytosis. It is a semi-synthetic derivative of staurosporine, an alkaloid from the bacterium Streptomyces staurosporeus, and is active in patients with mutations of CD135 (FMS-like tyrosine kinase 3 receptor, FLT3).[1]
After successful Phase II clinical trials, a Phase III trial for AML has started in 2008. It is testing midostaurin in combination with daunorubicin and cytarabine.[2] In another trial, the substance has proven ineffective in metastatic melanoma.[3]
In an open-label study of patients with mastocytosis-related organ damage (89 eligible patients meeting inclusion for the primary efficacy population), midostaurin showed efficacy in patients with advanced systemic mastocytosis, including the highly fatal variant mast-cell leukemia.[4]
References
- ↑ Fischer, T.; Stone, R. M.; Deangelo, D. J.; Galinsky, I.; Estey, E.; Lanza, C.; Fox, E.; Ehninger, G.; Feldman, E. J.; Schiller, G. J.; Klimek, V. M.; Nimer, S. D.; Gilliland, D. G.; Dutreix, C.; Huntsman-Labed, A.; Virkus, J.; Giles, F. J. (2010). "Phase IIB Trial of Oral Midostaurin (PKC412), the FMS-Like Tyrosine Kinase 3 Receptor (FLT3) and Multi-Targeted Kinase Inhibitor, in Patients with Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome with Either Wild-Type or Mutated FLT3". Journal of Clinical Oncology. 28 (28): 4339–4345. doi:10.1200/JCO.2010.28.9678. PMID 20733134.
- ↑ Clinical trial number NCT00651261 for "Daunorubicin, Cytarabine, and Midostaurin in Treating Patients With Newly Diagnosed Acute Myeloid Leukemia" at ClinicalTrials.gov
- ↑ Millward, M. J.; House, C.; Bowtell, D.; Webster, L.; Olver, I. N.; Gore, M.; Copeman, M.; Lynch, K.; Yap, A.; Wang, Y.; Cohen, P. S.; Zalcberg, J. (2006). "The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: a phase IIA clinical and biologic study". British Journal of Cancer. 95 (7): 829–834. doi:10.1038/sj.bjc.6603331. PMC 2360547
 . PMID 16969355.
. PMID 16969355. - ↑ Gotlib, Jason; Kluin-Nelemans, Hanneke C.; George, Tracy I.; Akin, Cem; Sotlar, Karl; Hermine, Olivier; Awan, Farrukh T.; Hexner, Elizabeth; Mauro, Michael J. (2016-06-29). "Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis". New England Journal of Medicine. 374 (26): 2530–2541. doi:10.1056/nejmoa1513098.