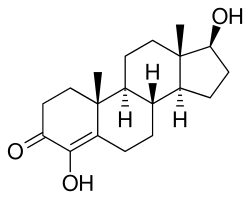
4-Hydroxytestosterone
 | |
 | |
| Identifiers | |
|---|---|
| |
| Synonyms | 4,17β-Dihydroxyandrost-4-en-3-one; Androst-4-ene-4,17β-diol-3-one |
| CAS Number |
2141-17-5 |
| PubChem (CID) | 160615 |
| DrugBank |
DB01485 |
| ChemSpider |
141138 |
| UNII |
912GOZ167T |
| Chemical and physical data | |
| Formula | C19H28O3 |
| Molar mass | 304.42 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
4-Hydroxytestosterone (4-OHT), also known as 4,17β-dihydroxyandrost-4-en-3-one, is a synthetic anabolic-androgenic steroid (AAS) and a derivative of testosterone that was never marketed. It was first patented by G.D. Searle & Company in 1955 and is testosterone with a hydroxy group at the four position. 4-OHT has moderate anabolic, mild androgenic, and anti-aromatase properties and is similar to the steroid clostebol (4-chlorotestosterone).
See also
References
| HMGCR | |
|---|---|
| FPS | |
| 24-DHCR24 |
|
| 20,22-Desmolase (P450scc) |
|
| 17α-Hydroxylase, 17,20-Lyase |
|
| 3α-HSD | |
| 3β-HSD | |
| 11β-HSD |
|
| 21-Hydroxylase |
|
| 11β-Hydroxylase |
|
| 18-Hydroxylase |
|
| 17β-HSD |
|
| 5α-Reductase |
|
| Aromatase |
|
| SST/EST |
|
| STS |
|
| 27-Hydroxylase | |
| Others |
|
See also: Androgenics • Estrogenics • Glucocorticoidics • Mineralocorticoidics • Progestogenics | |
This article is issued from Wikipedia - version of the 11/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.