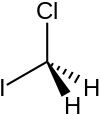
Chloroiodomethane
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chloro(iodo)methane | |
Other names
| |
| Identifiers | |
| 593-71-5 | |
| 3D model (Jmol) | Interactive image |
| 1730802 | |
| ChemSpider | 11154 |
| ECHA InfoCard | 100.008.915 |
| EC Number | 209-804-8 |
| PubChem | 11644 |
| |
| |
| Properties | |
| CH2ClI | |
| Molar mass | 176.38 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 2.422 g mL−1 |
| Boiling point | 108 to 109 °C (226 to 228 °F; 381 to 382 K) |
| Henry's law constant (kH) |
8.9 μmol Pa−1 kg−1 |
| Refractive index (nD) |
1.582 |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | WARNING |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| EU classification (DSD) |
|
| R-phrases | R36/37/38 |
| S-phrases | S26, S36 |
| NFPA 704 | |
| Related compounds | |
| Related alkanes |
|
| Related compounds |
2-Chloroethanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chloroiodomethane is a mixed liquid halomethane very soluble in acetone, benzene, diethyl ether and alcohol. Its refractive index is 1.5812 - 1.5832.
It crystallizes orthorhombic crystal system with space group Pnma with lattice constants: a = 6.383, b = 6.706, c = 8.867 (.10−1 nm).[1]
Chloroiodomethane is used in cyclopropanation (Simmon-Smith reaction), Mannich reaction, aminomethylation, epoxidation, ring opening and addition to terminal alkenes. It often replaces diiodomethane because of higher yields and selectivity.
References
- ↑ Torrie B. H.; Binbrek O. S.; von Dreele R. (1993). "Crystal structure of chloroiodomethane". Mol. Phys. 79 (4): 869–874(6). doi:10.1080/00268979300101691. Retrieved 2007-06-29.
External links
This article is issued from Wikipedia - version of the 9/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
