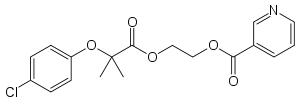
Etofibrate
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | C10AB09 (WHO) |
| Pharmacokinetic data | |
| Metabolism | Hydrolyzed to clofibric acid and niacin |
| Identifiers | |
| |
| CAS Number |
31637-97-5 |
| PubChem (CID) | 65777 |
| DrugBank |
DB08983 |
| ChemSpider |
59197 |
| UNII |
23TF67G79M |
| KEGG |
D07187 |
| ChEMBL |
CHEMBL358150 |
| ECHA InfoCard | 100.046.115 |
| Chemical and physical data | |
| Formula | C18H18ClNO5 |
| Molar mass | 363.792 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Etofibrate is a fibrate. It is a combination of clofibrate and niacin, linked together by an ester bond. In the body, clofibrate and niacin separate and are released gradually, in a manner similar to controlled-release formulations.[1]
References
- ↑ Sposito AC, Mansur AP, Maranhão RC, Rodrigues-Sobrinho CR, Coelho OR, Ramires JA (2001). "Etofibrate but not controlled-release niacin decreases LDL cholesterol and lipoprotein (a) in type IIb dyslipidemic subjects". Braz J Med Biol Res. 34 (2): 177–82. doi:10.1590/S0100-879X2001000200004. PMID 11175492. Free full text
This article is issued from Wikipedia - version of the 5/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.