Fipexide
 | |
| Clinical data | |
|---|---|
| ATC code | N06BX05 (WHO) |
| Identifiers | |
| |
| CAS Number |
34161-24-5 |
| PubChem (CID) | 3351 |
| ChemSpider |
3234 |
| UNII |
TG44VME01D |
| KEGG |
D07345 |
| ChEMBL |
CHEMBL254857 |
| ECHA InfoCard | 100.047.128 |
| Chemical and physical data | |
| Formula | C20H21ClN2O4 |
| Molar mass | 388.845 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
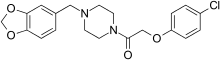
Fipexide (Attentil, Vigilor) is a psychoactive drug of the piperazine chemical class which was developed in Italy in 1983.[1] It was used as a nootropic drug in Italy and France, mainly for the treatment of senile dementia,[2] but is no longer in common use due to the occurrence of rare adverse drug reactions including fever[3] and hepatitis. Fipexide is similar in action to other nootropic drugs such as piracetam and is structurally similar to another more well-known nootropic, centrophenoxine. Chemically, it is an amide union of parachlorophenoxyacetate and methylenedioxybenzylpiperazine (MDBZP), and has been shown to metabolize to the latter, which plays a significant role in its effects.
See also
References
- ↑ Missale C, Pasinetti G, Govoni S, Spano PF, Trabucchi M. Fipexide: a new drug for the regulation of dopaminergic system at the macromolecular level. Bollettino Chimico Farmaceutico. 1983 Feb;122(2):79-85.
- ↑ Bompani R, Scali G. Fipexide, an effective cognition activator in the elderly: a placebo-controlled, double-blind clinical trial. Current Medical Research and Opinion. 1986;10(2):99-106.
- ↑ Guy C, Blay N, Rousset H, Fardeau V, Ollagnier M. Fever caused by fipexide. Evaluation of the national pharmacovigilance survey. Therapie. 1990 Sep-Oct;45(5):429-31.
This article is issued from Wikipedia - version of the 8/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.