Befuraline
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
41717-30-0 |
| PubChem (CID) | 68664 |
| ChemSpider |
61918 |
| UNII |
787AQ35GHR |
| ChEMBL |
CHEMBL1076256 |
| Chemical and physical data | |
| Formula | C20H20N2O2 |
| Molar mass | 320.385 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
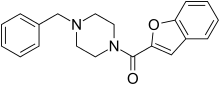
Befuraline (DIV-154) is a psychoactive drug and member of the piperazine chemical class which was developed in Germany in the 1970s.[1] Befuraline has stimulant and antidepressant effects and has seen some use in Germany and France, although it has never become widely used.[2] Befuraline's active metabolite benzylpiperazine is responsible for its effects.
See also
References
- ↑ Boksay IJ, Popendiker K, Weber RO, Soder A. Synthesis and pharmacological activity of befuraline (N-benzo[b]furan-2-ylcarbonyl-N'-benzylpiperazine), a new antidepressant compound. Arzneimittel-Forschung. 1979;29(2):193-204.
- ↑ Gastpar M, Gastpar G, Gilsdorf U. Befuraline, its safety and efficacy in depressed inpatients. Pharmacopsychiatry. 1985 Nov;18(6):351-5.
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
Stimulants (category) | |
|---|---|
| Adamantanes |
|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| Simple piperazines (no additional rings) | |
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.