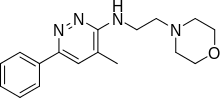
Minaprine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | N06AX07 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 2-2.5 hours |
| Identifiers | |
| |
| CAS Number |
25905-77-5 |
| PubChem (CID) | 4199 |
| ChemSpider |
4054 |
| UNII |
00U7GX0NLM |
| KEGG |
D05039 |
| ChEMBL |
CHEMBL278819 |
| ECHA InfoCard | 100.043.012 |
| Chemical and physical data | |
| Formula | C17H22N4O |
| Molar mass | 298.383 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| | |
Minaprine (INN, USAN, BAN) (brand names Brantur, Cantor) is a monoamine oxidase inhibitor antidepressant drug that was used in France for the treatment of depression until it was withdrawn from the market in 1996 because it caused convulsions.[1]
A study found that it acts as a reversible inhibitor of MAO-A (RIMA) in rats.[2] In a study it has also been found to weakly inhibit acetylcholinesterase in rat brain (striatum) homogenates.[3]
References
- ↑ Fung, M.; Thornton, A.; Mybeck, K.; Wu, J. H.-h.; Hornbuckle, K.; Muniz, E. (1 January 2001). "Evaluation of the Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999". Therapeutic Innovation & Regulatory Science. 35 (1): 293–317. doi:10.1177/009286150103500134.
- ↑ Kan JP, Mouget-Goniot C, Worms P, Biziere K (1986). "Effect of the antidepressant minaprine on both forms of monoamine oxidase in the rat". Biochemical Pharmacology. 35 (6): 973–978. doi:10.1016/0006-2952(86)90085-7. PMID 3954800.
- ↑ Contreras JM, Rival YM, Chayer S, Bourguignon JJ, Wermuth CG (1999). "Aminopyridazines as acetylcholinesterase inhibitors". Journal of Medicinal Chemistry. 42 (4): 730–741. doi:10.1021/jm981101z. PMID 10052979.
This article is issued from Wikipedia - version of the 9/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.