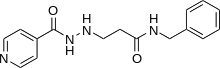
Nialamide
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | N06AF02 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
51-12-7 |
| PubChem (CID) | 4472 |
| DrugBank |
DB04820 |
| ChemSpider |
4317 |
| UNII |
T2Q0RYM725 |
| KEGG |
D07337 |
| ECHA InfoCard | 100.000.073 |
| Chemical and physical data | |
| Formula | C16H18N4O2 |
| Molar mass | 298.34 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Nialamide (Niamid, Niamide, Nuredal, Surgex) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class that was used as an antidepressant.[1] It was withdrawn by Pfizer several decades ago due to the risk of hepatotoxicity.[2][3]
The antiatherogenic activity of nialamide was used to design pyridinolcarbamate.[4]
See also
References
- ↑ William Andrew Publishing (1 December 2006). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- ↑ Shayne C. Gad (26 April 2012). Safety Pharmacology in Pharmaceutical Development: Approval and Post Marketing Surveillance, Second Edition. CRC Press. pp. 138–. ISBN 978-1-4398-4567-7.
- ↑ Edward Shorter (28 September 2008). Before Prozac : The Troubled History of Mood Disorders in Psychiatry: The Troubled History of Mood Disorders in Psychiatry. Oxford University Press. pp. 137–. ISBN 978-0-19-970933-5.
- ↑ https://books.google.co.uk/books?id=a1bmCAAAQBAJ&pg=PA387#v=onepage&q&f=false
This article is issued from Wikipedia - version of the 9/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.