Triprolidine
 | |
| Clinical data | |
|---|---|
| Trade names | Actidil, Myidil, Actifed (in the latter combined with pseudoephedrine and either dextromethorphan or guaifenesin) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | R06AX07 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 4% oral |
| Protein binding | 90% |
| Metabolism | Hepatic (CYP2D6) |
| Biological half-life | 4-6 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
486-12-4 |
| PubChem (CID) | 5282443 |
| IUPHAR/BPS | 1228 |
| DrugBank |
DB00427 |
| ChemSpider |
4445597 |
| UNII |
2L8T9S52QM |
| KEGG |
D01782 |
| ChEBI |
CHEBI:84116 |
| ChEMBL |
CHEMBL855 |
| ECHA InfoCard | 100.006.934 |
| Chemical and physical data | |
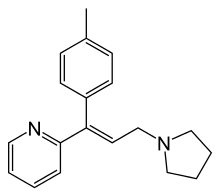
| Formula | C19H22N2 |
| Molar mass | 278.391 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 60 °C (140 °F) |
| Solubility in water | 500 mg/mL (20 °C) |
| |
| |
| | |
Triprolidine is an over-the-counter antihistamine with anticholinergic properties.[1] It is used to combat the symptoms associated with allergies and is sometimes combined with other cold medications designed to provide general relief for flu-like symptoms.[2] Like many antihistamines, the most common side effect is drowsiness.[1] Triprolidine is a quick acting drug that can clear congestion and stop runny noses in 15–30 minutes.
References
- 1 2 Goldsmith, P.; Dowd, P. M. (1993). "The new H1 antihistamines. Treatment of urticaria and other clinical problems". Dermatologic clinics. 11 (1): 87–95. PMID 8094649.
- ↑ Williams, B. O.; Liao, S. H.; Lai, A. A.; Arnold, J. D.; Perkins, J. G.; Blum, M. R.; Findlay, J. W. (1984). "Bioavailability of pseudoephedrine and triprolidine from combination and single-ingredient products". Clinical pharmacy. 3 (6): 638–643. PMID 6509877.
| Aminoalkyl ethers |
|
|---|---|
| Substituted alkylamines | |
| Substituted ethylenediamines |
|
| Phenothiazine derivatives | |
| Piperazine derivatives | |
| Others for systemic use |
|
| For topical use | |
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (inhibitors) |
| ||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||
| Others |
| ||||||||||||||
This article is issued from Wikipedia - version of the 10/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.