Telapristone
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
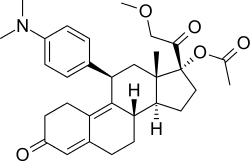
| Synonyms | CDB-4124; 17α-Acetoxy-21-methoxy-11β-[4-N,N-dimethylaminophenyl]-19-norpregna-4,9-diene-3,20-dione) |
| CAS Number | 198414-31-2 |
| PubChem (CID) | 9806190 |
| ChemSpider |
7981950 |
| UNII |
1K9EYK92PQ |
| Chemical and physical data | |
| Formula | C31H39NO5 |
| Molar mass | 505.65 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Telapristone (INN), as telapristone acetate (proposed trade names Proellex, Progenta; former code name CDB-4124), is an investigational selective progesterone receptor modulator (SPRM) being studied for the treatment of certain progesterone-sensitive conditions.[1] It was originally developed by the National Institutes of Health (NIH), and as of 2012, is in phase II clinical trials for the treatment of uterine fibroids and endometriosis.[2] In addition to its actions as an SPRM, telapristone also has some antiglucocorticoid activity.
See also
References
- ↑ Attardi BJ, Burgenson J, Hild SA, Reel JR (2004). "In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone". J Steroid Biochem Mol Biol. 88 (3): 277–88. doi:10.1016/j.jsbmb.2003.12.004. PMID 15120421.
- ↑ ClinicalTrials.gov
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.