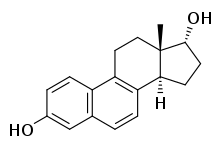
17α-Dihydroequilenin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Identifiers | |
| |
| Synonyms | NSC-12171 |
| CAS Number | 6639-99-2 |
| PubChem (CID) | 223998 |
| ChEBI | CHEBI:34178 |
| ChEMBL | CHEMBL135236 |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.3502 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
17α-Dihydroequilenin, or α-dihydroequilenin, also known as 6,8-didehydro-17α-estradiol, as well as estra-1,3,5(10),6,8-pentaen-3,17α-diol, is a naturally occurring steroidal estrogen found in horses that is closely related to equilin, equilenin, and 17α-estradiol, and, as the 3-sulfate ester sodium salt, is a minor constituent (1.2%) of conjugated equine estrogens (Premarin).[1]
See also
References
- ↑ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
| ER |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPER |
| ||||||||||
See also: Androgenics • Glucocorticoidics • Mineralocorticoidics • Progestogenics • Steroid hormone metabolism modulators | |||||||||||
This article is issued from Wikipedia - version of the 11/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.