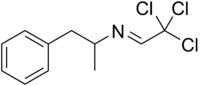
Amfecloral
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms |
alpha-methyl-N-(2,2,2-trichloroethylidene)phenethylamine; N-(2,2,2-trichloroethylidene)amphetamine |
| CAS Number | 5581-35-1[1] |
| PubChem (CID) | 21759 |
| ChemSpider |
20451 |
| UNII |
6015XOA0BI |
| KEGG |
D02926 |
| ChEMBL |
CHEMBL2105544 |
| Chemical and physical data | |
| Formula | C11H12Cl3N |
| Molar mass | 264.58 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Amfecloral (INN), also known as amphecloral (USAN), is a stimulant drug of the phenethylamine and amphetamine chemical classes that was used as an appetite suppressant under the trade name Acutran, but is now no longer marketed.[1] It was classified as an anorectic drug with little to no stimulant activity in a 1970 review.[2] The British Pharmacopoeia Commission approved the name in 1970.[3] The raw ingredients used in manufacturing it were d-amphetamine and chloral hydrate.[4]
See also
References
- 1 2 Ganellin, C. R.; Triggle, David J. (1996). Dictionary of pharmacological agents, Volumes 1-2. Chapman & Hall. p. 67. ISBN 9780412466304.
- ↑ Van Rossum, JM (1970). "Mode of action of psychomotor stimulant drugs.". International review of neurobiology. 12: 307–83. PMID 4918147.
- ↑ "Notes and News". The Lancet. 296 (7675): 730–732. October 1970. doi:10.1016/S0140-6736(70)92010-6.
- ↑ McPherson, Edwin M. (2007). Pharmaceutical Manufacturing Encyclopedia. (3rd ed. ed.). Burlington: Elsevier. p. 244. ISBN 9780815518563. CS1 maint: Extra text (link)
| GABAA |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAB | |||||||||||||||||||||||||||||||||||||||||||
| H1 |
| ||||||||||||||||||||||||||||||||||||||||||
| α2-Adrenergic | |||||||||||||||||||||||||||||||||||||||||||
| 5-HT2A |
| ||||||||||||||||||||||||||||||||||||||||||
| Melatonin | |||||||||||||||||||||||||||||||||||||||||||
| Orexin | |||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
| α1 |
| ||||||
|---|---|---|---|---|---|---|---|
| α2 |
| ||||||
| β |
| ||||||
| |||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
See also: GHBergics • Glutamatergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia - version of the 10/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.