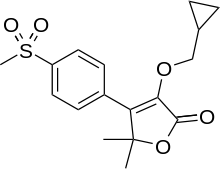
Firocoxib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Equioxx; Previcox |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATCvet code | QM01AH90 (WHO) |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | 189954-96-9 |
| PubChem (CID) | 208910 |
| ChemSpider |
181008 |
| UNII |
Y6V2W4S4WT |
| KEGG |
D03712 |
| ChEBI | CHEBI:76136 |
| ChEMBL |
CHEMBL69998 |
| Chemical and physical data | |
| Formula | C17H20O5S |
| Molar mass | 336.402 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Firocoxib (INN; brand names Equioxx and Previcox) is a non-steroidal anti-inflammatory drug of the COX-2 inhibitor (coxib) class, currently approved for use in dogs and horses. Firocoxib was the first COX-2 inhibitor approved by the U.S. Food and Drug Administration for horses.[1] Firocoxib is not intended or approved for use in human medicine.[2]
References
- ↑ "New NSAID Equioxx (firocoxib) Approved by USEF". The Horse. July 2, 2007. Retrieved 2008-04-18.
- ↑ Equioxx, European Medicines Agency
This article is issued from Wikipedia - version of the 4/19/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.