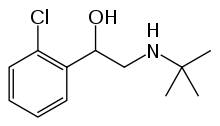
Tulobuterol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Inhaled, oral |
| ATC code | R03AC11 (WHO) |
| Identifiers | |
| |
| CAS Number |
41570-61-0 |
| PubChem (CID) | 5606 |
| ChemSpider |
5404 |
| UNII |
591I9SU0F7 |
| KEGG |
D02151 |
| ChEMBL |
CHEMBL1159717 |
| ECHA InfoCard | 100.168.691 |
| Chemical and physical data | |
| Formula | C12H18ClNO |
| Molar mass | 227.730 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tulobuterol (INN) is a long-acting beta2-adrenergic receptor agonist, marketed in Japan as a transdermal patch under the name Hokunalin tape (ホクナリンテープ).[1]
Currently, it is only legal in 7 countries: Japan, Germany, China, South Korea, Bangladesh, Pakistan, and Venezuela.
References
- ↑ Horiguchi, T.; Kondo, R.; Miyazaki, J.; Fukumokto, K.; Torigoe, H. (2011). "Clinical Evaluation of a Transdermal Therapeutic System of the ß2-Agonist Tulobuterol in Patients with Mild or Moderate Persistent Bronchial Asthma". Arzneimittelforschung. 54 (5): 280–285. doi:10.1055/s-0031-1296971. PMID 15212190.
This article is issued from Wikipedia - version of the 6/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.