Talipexole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Domin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | Alefexole |
| CAS Number |
36085-73-1 |
| PubChem (CID) | 5374 |
| IUPHAR/BPS | 5442 |
| ChemSpider |
5181 |
| UNII |
7AM2J46Z1Y |
| ChEMBL |
CHEMBL1289023 |
| Chemical and physical data | |
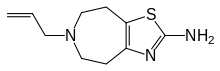
| Formula | C10H15N3S |
| Molar mass | 209.31 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Talipexole (B-HT920, Domnin) is a dopamine agonist that is marketed as a treatment for Parkinson's Disease in Japan by Boehringer Ingelheim; it was introduced in 1996.[1] As of December 2014 it was not approved for marketing in the US nor in Europe.[2]
Talipexole is a D2 dopamine receptor agonist and interacts both pre- and post-synaptic receptors. It also is an α2-adrenergic agonist.[3]
The main side effects are drowsiness, dizziness, hallucinations and minor gastrointestinal complaints.[3] In 2008 the Japanese Ministry of Health, Labour, and Welfare mandated that Boehringer add a warning to the label concerning the risk of sudden onset of sleep.[4]:15
References
- ↑ PharmaLetter 22 July 1996 First Launch In Japan For Talipexole
- ↑ EvaluatePharma Database. Page accessed 9 December 2014
- 1 2 Benkert O, et al. Dopamine agonists in schizophrenia: a review. Eur Neuropsychopharmacol. 1995;5 Suppl:43-53. PMID 8775758
- ↑ Japanese Ministry of Health, Labour and Welfare March 2008 Pharmaceuticals and Medical Devices Safety Information No. 245
This article is issued from Wikipedia - version of the 1/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.