Aceburic acid
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
26976-72-7 |
| PubChem (CID) | 176865 |
| ChemSpider |
154039 |
| UNII |
F777XEP0LL |
| ChEMBL |
CHEMBL2104034 |
| Chemical and physical data | |
| Formula | C6H10O4 |
| Molar mass | 146.141 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
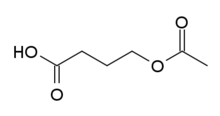
Aceburic acid (INN), also known as 4-acetoxybutanoic acid or 4-hydroxybutyric acid acetate, is drug described as an analgesic which was never marketed.[1] It is the acetyl ester of gamma-hydroxybutyrate (GHB, which is 4-hydroxybutanoic acid),[1] and based on its structural relation to GHB, is likely to behave as a prodrug to it.
See also
- 1,4-Butanediol (1,4-BD)
- γ-Butyrolactone (GBL)
- γ-Hydroxybutyraldehyde (GHBAL)
- γ-Valerolactone (GVL)
- Aceturic acid
- Aceglutamide
References
- 1 2 C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1052–. ISBN 978-0-412-46630-4.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
See also: GHBergics • Glutamatergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) | |||||||||||||||||||
| Enzyme (inhibitors) | |||||||||||||||||||
| Others | |||||||||||||||||||
See also: GABAergics • Glutamatergics • Glycinergics | |||||||||||||||||||
This article is issued from Wikipedia - version of the 9/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.