Binedaline
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
60662-16-0 |
| PubChem (CID) | 42509 |
| ChemSpider |
38769 |
| UNII |
3AVG9P140R |
| ChEMBL |
CHEMBL2104611 |
| Chemical and physical data | |
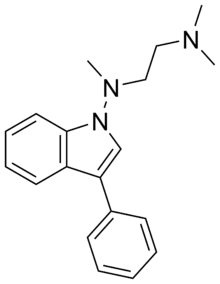
| Formula | C19H24ClN3 |
| Molar mass | 329.86696 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Binedaline (Ixprim), also called binodaline, is a drug that was being investigated as an antidepressant in the 1980s but never marketed.[1][2] It acts as a selective norepinephrine reuptake inhibitor (Ki = 25 nM), with relatively insignificant influence on the serotonin (Ki = 847 nM) and dopamine (Ki >= 2 µM) transporters.[3] It has negligible affinity for the α-adrenergic, mACh, H1, or 5-HT2 receptors.[3]
References
- ↑ Faltus; Geerling, F. (1984). "A controlled double-blind study comparing binedaline and imipramine in the treatment of endogenous depression". Neuropsychobiology. 12 (1): 34–38. doi:10.1159/000118107. PMID 6239991.
- ↑ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- 1 2 Morin; Zini, R.; Urien, S.; Tillement, J. P. (1989). "Pharmacological profile of binedaline, a new antidepressant drug". The Journal of Pharmacology and Experimental Therapeutics. 249 (1): 288–296. PMID 2540319.
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
Stimulants (category) | |
|---|---|
| Adamantanes |
|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| α1 |
| ||||||
|---|---|---|---|---|---|---|---|
| α2 |
| ||||||
| β |
| ||||||
| |||||||
This article is issued from Wikipedia - version of the 10/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.