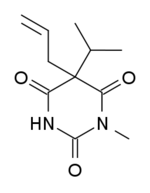
Enallylpropymal
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| Synonyms | Enallylpropymal |
| CAS Number |
1861-21-8 |
| PubChem (CID) | 95636 |
| ChemSpider | 86329 |
| ECHA InfoCard | 100.015.876 |
| Chemical and physical data | |
| Formula | C11H16N2O3 |
| Molar mass | 224.256 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Enallylpropymal (Narconumal) is a barbiturate derivative developed by Hoffman la Roche in the 1930s.[1] It has sedative and hypnotic effects and is considered to have a moderate abuse potential.[2]
References
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: GABAergics | |
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.